Blog In a Scientist’s Words: Why Agriculture Needs Gene Editing

By Andrew Wight
Gene editing, driven by CRISPR technology, has accelerated the breeding of sustainable and resilient crop varieties. But outside of laboratories and plant breeding stations, few people are aware of exactly what gene editing consists of, how it differs from genetically modified organisms (GMOs), and the role of gene edited crops in sustainably feeding Earth's population in a changing climate. Colombian-Australian science journalist Andrew Wight sat down with Colombian geneticist Paul Chavarriga, from the Alliance of Bioversity International and CIAT, to find some answers over local coffee and Caleño-style pandebono in Cali, Colombia.
Andrew Wight: So what exactly are these technologies, and how are they different from GMOs?
Paul Chavarriga: For gene editing,CRISPR technology allows us to get into a plant genome and change a simple gene to produce a new trait. For example, if you have a plant that’s susceptible to bacterial disease, with a knockout you can make the plant resistant to that particular disease, making it more productive for farmers.
We're not inventing anything that nature hasn’t already invented; in plant breeding, we speed up the same process of genetic changes that gives you better characteristics in key crops. The outcome is the same as what you're trying to get through mutagenesis or traditional plant breeding, but it's more precise, because you know which gene you're targeting.
At the Alliance, our ears are tuned to identify technologies across the world that allow us to have a positive impact on modern agriculture, and gene editing is a prime example, as this technology allows us to rapidly achieve the precise result we’re looking for, simply by making a small change to the genome. However, we still need to speed up this process, because with increasing food insecurity, we can no longer afford to take 10-20 years to develop new varieties.

Plant geneticist Paul Chavarriaga. Photo: E. Ramirez
Andrew: Can you give me an example? I heard you’re currently working on heavy metal uptake in cacao, the main ingredient in chocolate.
Paul: Some of the world’s best cacao “fino de aroma” comes from Colombia, but the soil here is (naturally) high in cadmium in several cacao plantations, a heavy metal that in large quantities is toxic for humans. The European Union has set limits on the concentration of cadmium in the cacao they import, so we are at risk of exports being rejected, which has already happened, and can impact farmers’ incomes.
To solve this, we’re trying to shut down the single gene that we know is responsible for absorbing cadmium from the soil though the roots. This gene sits in the roots in the form of a pump that introduces cadmium along with other metals. Fixing this through traditional breeding (from zero to a new variety) may take about 20 years, but today, we know that editing this gene changes the amount of cadmium that the cacao tree absorbs.
More on cadmium in cacao
Andrew Wight: For decades, many consumers were frightened by “Frankenfoods” like the GMO tomato with a fish gene in it. How has this impacted gene editing?
Paul: Back then, there was very little public information about how GMOs were made, but today I think this is different, and people are getting more informed. In terms of the controversy of GMOs, I'll put it plainly: it was misinformation in terms of GMOs being poisonous; still is.
Today there's so much demand for more productive, environment-friendly agriculture, and on top of it, you have the rapidly increasing impacts of climate change. Our role is to explore as many alternatives as possible, as long as they are safe — and products are safe if we are basically imitating life or reproducing what is already in nature and what you already eat.
In 2012, CRISPR was just coming out. We knew it was going to be difficult, and the negative GMO stories were still around, but we decided that we needed to invest in this all the same. By 2020, CRISPR had won a Nobel Prize, and our projects were well underway.
A major difference with GMOs is that Gene Edited products do not necessarily possess a novel combination of genetic material in the final product. Gene editing allows you to make specific changes to genes that already exist in the genome, changes that may mimic those that are in the germplasm of the species of interest.

Researchers from the Alliance's gene-editing platform at the Americas Hub, Colombia.
Andrew: So to wrap up, what’s your outlook on the future of this technology?
Paul: I hope that people’s perceptions continue to change. In Latin America, Canada, the USA and several other countries across the world, regulators have adopted a more pragmatic approach, distinguishing between gene editing and GMOs.
From a scientific perspective, this technology is relatively simple to apply and it’s evolving rapidly. For example, since 2020, Colombia has declared seven products as conventional, non-GMO, among them waxy corn, rice tolerant to leaf bacterial blight, and pigs resistant to the PRRS (porcine reproductive and respiratory syndrome), products that may soon be part of the Colombians’ food chain.
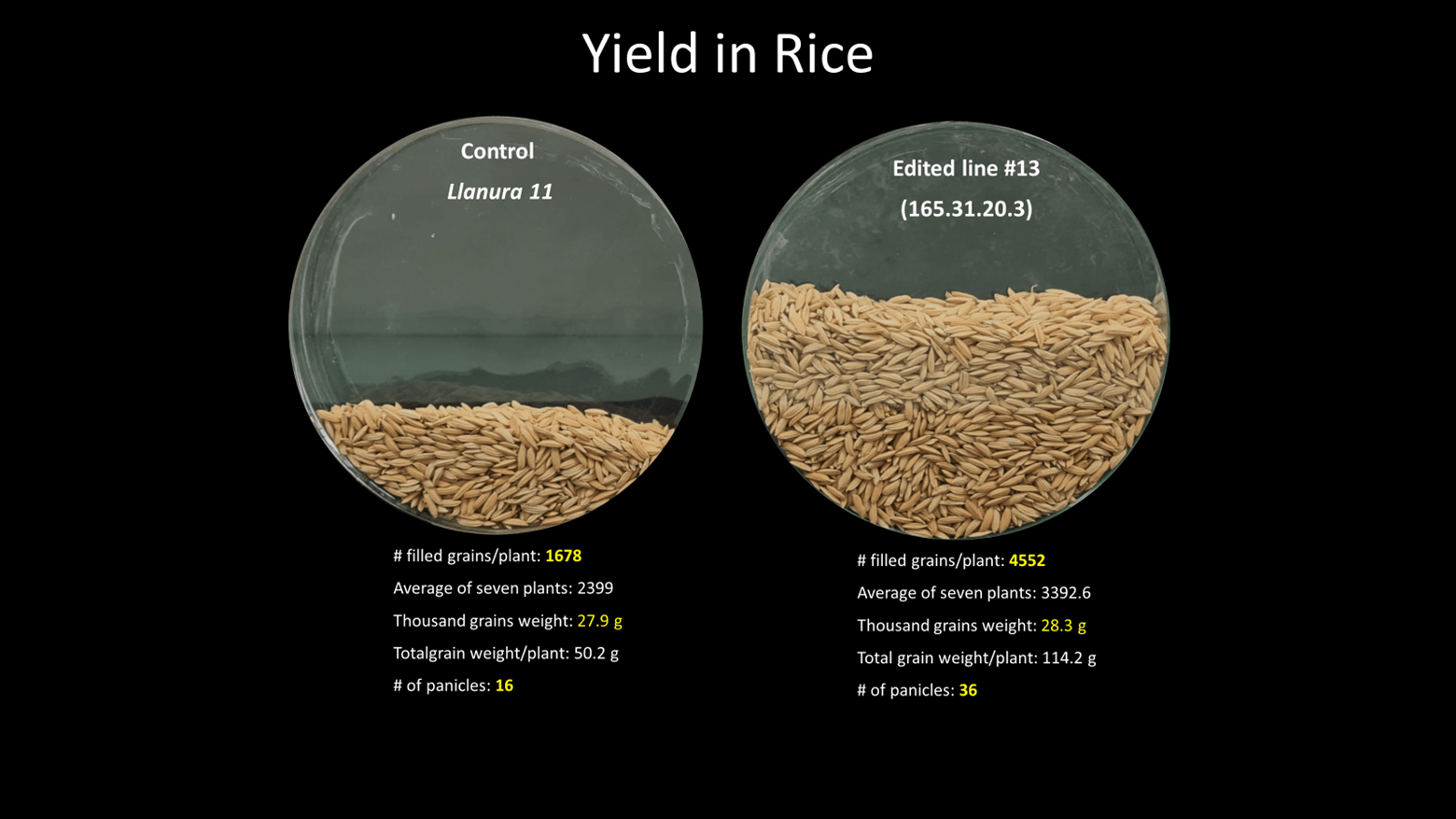
In the case of the Alliance of Bioversity International and CIAT, we have a new line of rice that has been edited to double its productivity. With these new varieties, on the same piece of land, of the same size, with the same inputs, you may harvest twice as much rice as before!
This technology really is a geneticist’s dream come true, and can make huge contributions to sustainable food systems around the world.
Fast Facts: Gene Editing
What is gene editing?
Gene editing uses a technology called CRISPR and an enzyme called Cas9 (There are many Cas-like enzymes) to make very precise changes at very exact places in a genome, for example, making a change that will inactivate a gene. Gene editing is also very cost effective compared to previous technologies.
How is gene editing different from genetically modified organisms (GMOs)?
Most GMOs in use are products of transgenic modification, where foreign DNA from a different species is introduced into the plant. But gene editing’s precision and versatility is mostly used to “flip a switch”, such as shutting off or down a gene that, for instance, absorbs heavy metals into a cacao plant.
Opportunities
Together with traditional plant breeding, gene editing is able to develop safer, environment-friendly, more productive crops that can be quickly deployed to respond to a rapidly changing climate, new diseases and productivity challenges. The Alliance’s high-throughput phenotyping facilities, which includes drones and data analytics (Pheno-i), are identifying targets for gene editing.
Challenges and Concerns
In the 1990s, an antifreeze gene from a fish was spliced into tomato cells to make the tomato freeze resistant. These kinds of experiments caught media attention, and raised scientifically unsustained fears amongst the public and with regulators, resulting in restrictions on GMOs, especially in the European Union. Although gene editing is more precise and safer than GMOs, consumers continue to voice concerns of adapted varieties. However, ongoing dissemination of scientific information among consumers, regulators and the general public has meant that more people understand the uses of gene-editing compared to GMOs. This may be the main reason why countries have made efforts to regulate it without hindering its development and use.
Gene Editing Projects: Alliance of Bioversity International and CIAT
- Rice: The Alliance has tested in the field 20 different gene-edited, transgene-free rice varieties, with resistance to leaf bacterial blight, showing that their phenotype, including yield, resembles that of their non-gene-edited counterparts. Similarly, a Colombian rice variety (Llanura 11), edited to increase grain number has also been tested in the field to confirm that it produces twice as much grains/plant than its non-gene-edited counterpart.
- Cacao: The best cacao trees are typically grown in soils with high levels of cadmium (a heavy metal), so scientists are aiming to knock out the molecular pump that absorbs this heavy metal into the plant.
- Beans: More easily digested beans are being developed by knocking out pathways that produce indigestible polysaccharides.
- Cassava: The Alliance has produced waxy (low amylose/high amylopectin) cassava using CRISPR to mimic natural mutations of commercial cultivars. After one cycle of sexual reproduction, these new waxy lines are transgene-free (non-GMO).
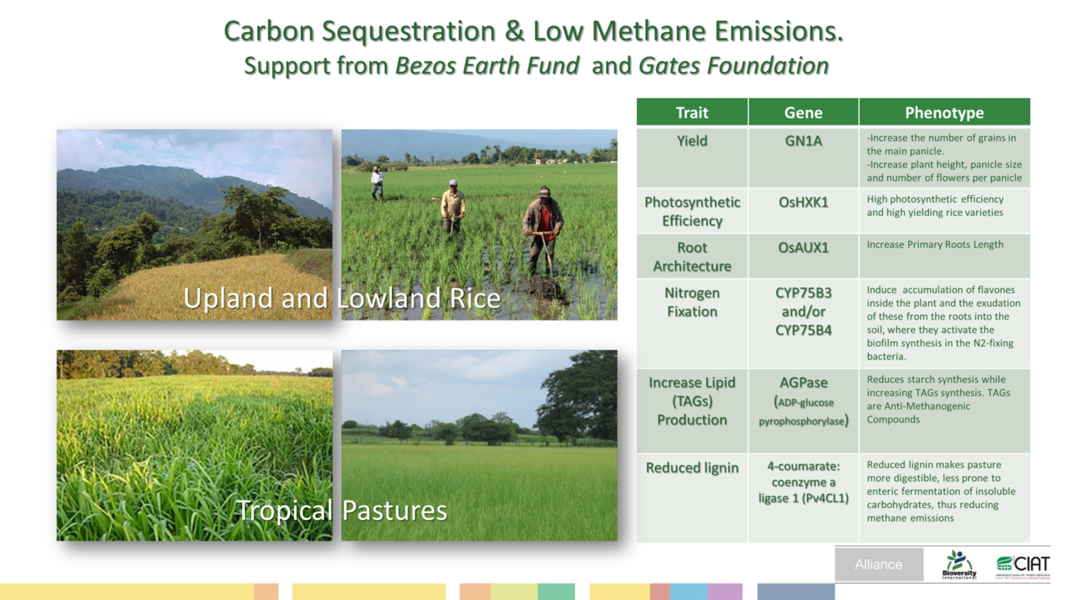
Carbon Sequestration & Low Methane in Rice and Forages